Deposition of Reactive Materials
Thin films of reactive materials should be deposited in a highly controlled environment to prevent contaminating the resulting layer and achieve a pure coating with the required properties. Some deposition source materials are highly resistant to forming compounds with other materials, which are called noble metals like gold. In contrast, others can be very sensitive to the deposition chamber environment.
Here, we will discuss more details on the deposition of reactive materials and the keys to obtain a clean coating.
What Are Reactive Materials?
Most reactive materials have a high affinity to oxygen to form an oxide compound (Burning reaction) in oxygen-containing environments. Deposition of such materials should be performed under high vacuum conditions since a small trace of oxygen can hinder the goal of coating a pure layer.
Also, some materials have catalytic reactions with some ingredients of the air, like H2 or CO, which makes them sensible to small amounts of these compounds. Meanwhile, such reactions may contaminate the deposition source, the deposition chamber should get degassed from any reactive substance by using a high vacuum chamber and inert gas injection.
Classification of Reactive Materials Deposition
Noble Metals
Noble metals, the metallic chemical elements that are generally resistant to corrosion, such as gold, silver, and platinum, have the least preference to react with oxygen, according to their Standard Reduction Potential (SRP) number, for their full d-subshells. The higher the SRP, the lower the oxidation possibility (Table 1).
| Element | SRP(V) |
| Gold (Au) | 1.5 |
| Pt | 1.2 |
| Ir | 1.16 |
| Pd | 0.915 |
| Os | 0.85 |
| Hg | 0.85 |
| Rh | 0.8 |
| Ag | 0.79 |
| Ru | 0.6 |
Electronic Properties of Metals
There are other metals also known as noble, like palladium, ruthenium, rhodium, osmium, and iridium that have a full d-subshell in the atomic state but in condensed form have a partially filled sp band at the expense of d-band occupancy. This causes their surface to be covered by carbon monoxide quickly if not kept under high vacuum conditions and gives rise to notable catalytic applications of such materials.
Alkali Metals
Due to their electron affinity, materials have a different tendency to react with oxygen. Substances like alkali metals easily react with oxygen when exposed to air (Figure 1).
Lithium is a highly reactive alkali metal that is widely used to produce rechargeable batteries that are used in smartphones, tablets, etc., whose deposition process should be executed in a high vacuum chamber.
Lithium Deposition
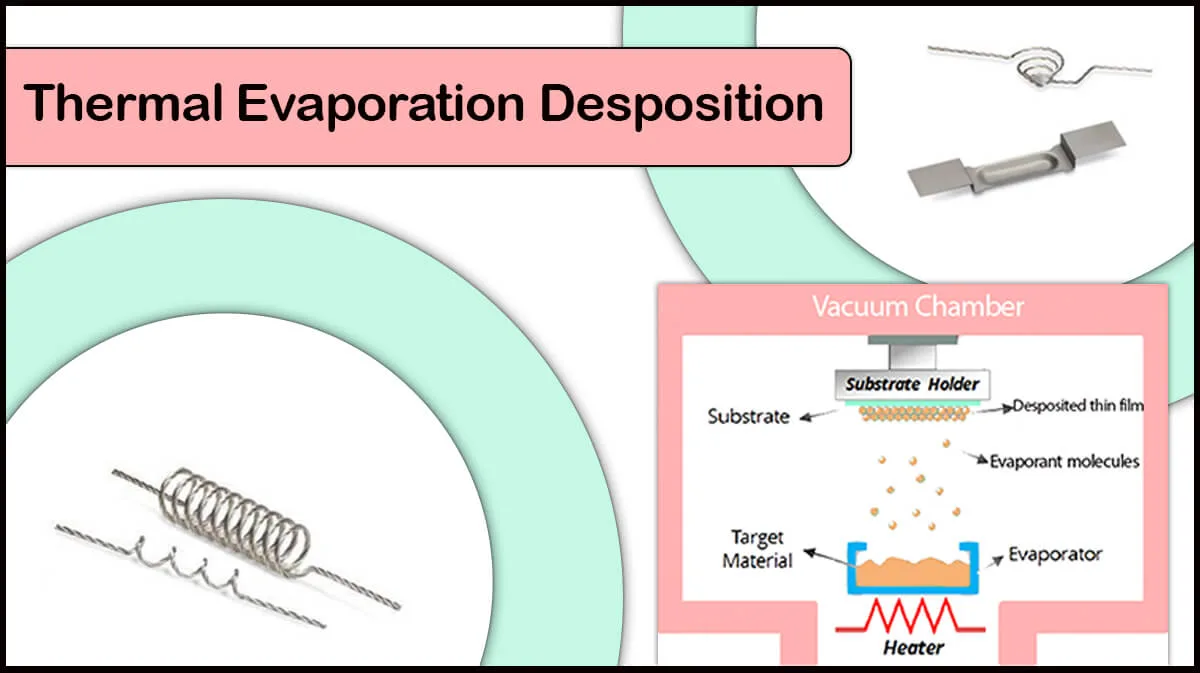
The best way for lithium deposition with a controllable deposition rate is the thermal evaporation method. Deposition of this metal through other techniques, such as sputtering or electron gun can be challenging due to its low melting point since it is very difficult to control the deposition rate.

Thermal Evaporation of Reactive Metals
In the process of thermal evaporation deposition of materials, the evaporant source should be heated enough to melt down, and then evaporate and coat the substrate. When an oxide layer is formed on evaporants like lithium, higher power is needed to break the oxide layer (Which is not thermally conductive) and melt the confined metal beneath. This extreme heat triggers flash evaporation of lithium, leading to uncontrolled deposition of the source material.
To prevent oxidation, lithium must be placed in the environment of inert argon gas or oil, and its deposition chamber should be highly vacuumed from oxygen. To this end, Vac Coat Ltd. has made it possible for the DTT and DTE thermal evaporators to be able to integrate with a Glovebox to prevent the oxidation of materials such as lithium. If you want to evaporate highly reactive metals such as lithium by the thermal evaporation method, be sure to see the specifications of the DTT and DTE thermal evaporation deposition systems produced by Vac Coat.
Surface Reactivity of Sputter Targets
Metal Targets’ Surface Reactivity
Sputtering target specifications should be considered thoroughly before initiating a sputter deposition process. In the case of using metal targets, the purity of the coated film is vital, so the study of the surface reactivity of the targets before utilizing them is essential.
Noble Metal Sputtering Targets Reactivity
According to the resistance to the reactivity of the target material, the target maintenance and cleaning process, as well as the required vacuum level inside the deposition chamber differ. Surfaces of “physically defined” noble metals (e.g., gold) can be easily cleaned and kept clean for a long time, while more reactive metal surfaces, such as those of platinum or palladium, are covered by carbon monoxide very quickly under low vacuum conditions.
Case Study: Carbon Compounds Inside the Pd Sputtering Chamber
In a research done by S. Fuchs, et al., the reaction rate of Pt, Pd, and Rh noble metals in the form of polycrystalline foils, ribbons, and wires was studied by measuring catalyzed oxidation of CO by O2. The reaction rate values measured at total pressures exceeding 1 mbar correspond to the highest activities on noble metal catalysts like Pt, Pd, and Rh, as shown in Figure 2.

An increase in the CO pressure (Hence the base pressure) and Pd (Target) temperature raises the reaction rate. These conditions give rise to the enhanced carbon-carbon bonding reactions facilitated by the presence of the palladium target as the catalyst. As a result, a black residue slightly covers the target dark shield, sample, and the chamber which can be attributed to the carbon compounds formed by Pd inside the high-temperature chamber.
Oxidizing Metals
Oxidizing metals like aluminum, tungsten, and chromium are also sensitive to oxygen-containing environments that prevent the production of a thin film of pure metallic film under low vacuum conditions. The formation of a thin oxide layer on the target surface before starting the deposition process, or reaction of the sputtered target material during the coating process, results in forming a poorly conductive layer, which is undesirable. High vacuum DST1 and DSCT coaters are ideal for a clean and precise deposition of oxidizing metals.
Case Study: Aluminum Sputtering
Aluminum sputter targets can be used in various sputtering techniques, such as DC dipole sputtering, RF sputtering, ion beam sputtering, and magnetron sputtering to produce coatings for different purposes, like reflective coatings, conductive films, semiconductor thin films, capacitor films, decorative and protective coatings, integrated circuits, displays, etc.
Aluminum is widely used due to its lower price compared to other sputtering targets. Al is an oxidizing metal, so the deposition process for aluminum target deposition should take place under high vacuum conditions, with a base pressure of about 10-5 Torr.
The coating process should include a preliminary pre-sputtering step with a closed shutter to shield the substrate from the flow of plasma.
During the pre-sputtering process, it is recommended to gradually increase the plasma current to the desired level to remove the oxide layer and maintain a stable plasma for a few minutes. When the target surface is cleaned the deposition process can initiate.

Importance of Surface Reactivity in Deposition
Understanding the surface reactivity of sputtering targets is critical for achieving high-purity and high-quality thin films in the deposition processes. Noble metals such as gold and silver exhibit low surface reactivity and are relatively easy to maintain and can be deposited under even low vacuum conditions, which is achievable in Vac Coat sputter coaters like DSR1 and DSCR.
Other more reactive metals targets like palladium or platinum require strict environmental controls and higher vacuum conditions, as provided in Vac Coat high vacuum sputter coaters, including DST1 and DSCT models, due to their catalytic behavior and tendency to adsorb impurities in the chamber atmosphere, such as oxygen and CO.
Oxidizing metals, including aluminum and chromium, are prone to forming insulating oxide layers. So, deposition of reactive materials, like Al, necessitates high vacuum levels and careful pre-sputtering procedures to ensure a high-quality thin film deposition.
Moreover, thermal evaporation deposition of highly reactive lithium sources is made possible by Vac Coat DTT and DTE models. Vac Coat offers tailored sputtering setup and chamber conditions to meet the specific chemical properties of different target materials, so as to minimize contamination, ensure film purity, and improve process efficiency. Also, Vac Coat multi-cathode sputter coaters (Can include thermal evaporator), like DST2-TG and DST3 help to maintain the vacuum level when switching over deposition of different source materials.
References
- S. Fuchs, T.Hahn, H.G. Lintz, “The oxidation of carbon monoxide by oxygen over platinum, palladium and rhodium catalysts from 10−10 to 1 bar”, Chemical engineering and processing, 1994, V 33(5), pp. 363–369.
- https://www.sputtertargets.net/an-overview-of-aluminum-sputtering-target.html
- https://studyrocket.co.uk/revision/gcse-chemistry-combined-science-ocr/reactions-and-products/reactivity-of-metals