Chemical Vapor Deposition (CVD)
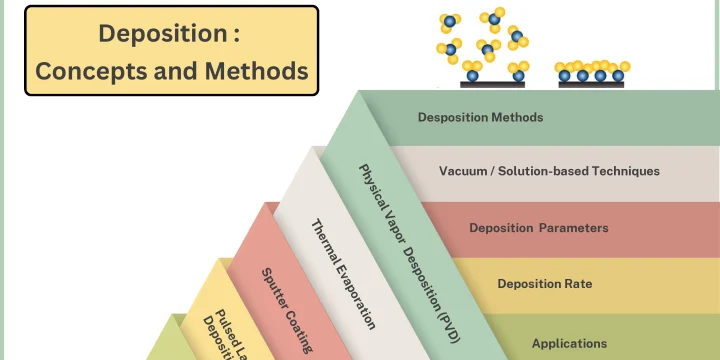
[vc_row][vc_column width="3/4"][vc_row_inner][vc_column_inner][vc_column_text css=""] Chemical Vapor Deposition (CVD) Chemical Vapor Deposition (CVD) is a chemical process used to create layers with different applications on different surfaces. In this deposition method, the desired surface (substrate) is exposed to the vapor of one or more chemicals. Then, in order to create a solid layer with the desired chemical composition, the gas atoms in the chamber decompose on the surface of the substrate or react with each other chemically. This deposition method is classified in different ways depending on what chemical method it starts with. [/vc_column_text][/vc_column_inner][/vc_row_inner][vc_row_inner css=".vc_custom_1735825376632{background-color: #D9D9D9 !important;}"][vc_column_inner width="1/2"][vc_single_image image="11849" img_size="large" alignment="center" style="vc_box_outline" border_color="mulled_wine" onclick="custom_link" css="" link="https://vaccoat.com/blog/deposition/"][/vc_column_inner][vc_column_inner width="1/2"][vc_column_text css=""] Deposition is a set of processes used to create thin or thick layers of a substance atom-by-atom or molecule by molecule on a solid…