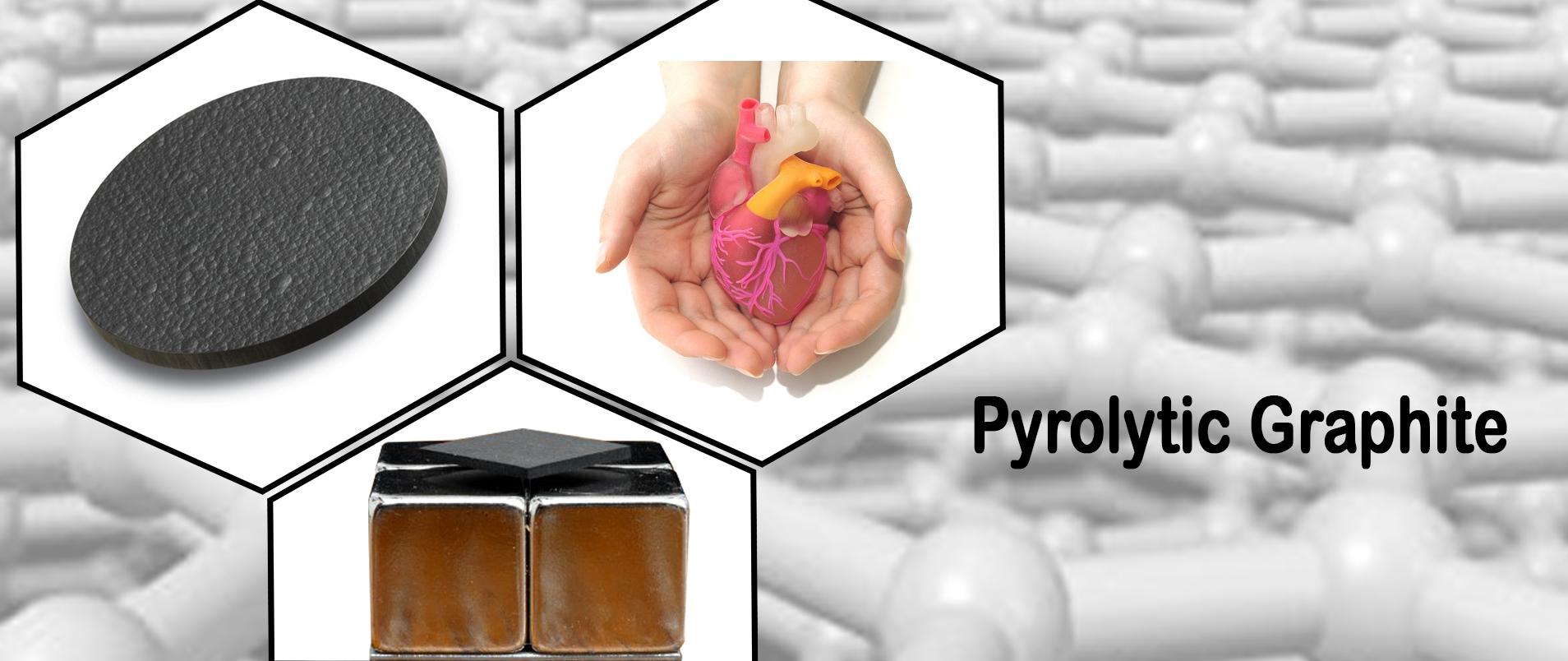
Pyrolytic Graphite
Pyrolytic graphite is a polycrystalline form of graphite that is deposited from the vapor phase by thermal decomposition of a simple hydrocarbon such as methane. Pyrolytic graphite is a man-made material similar to graphite, except that there are covalent bonds between its graphene layers. Graphite has a layered structure, and each carbon atom in each layer is bonded to three adjacent atoms. The atomic structure of graphite is a two-dimensional lattice of hexagons whose first and third layers exactly match each other, while the second layer is slightly displaced relative to these two layers.
Pyrolytic Graphite Structure
But the material deposited as pyrolytic graphite consists of layers of wavy, twisted plans composed of carbon atoms arranged in a hexagonal structure. These layers are mutually parallel to each other but rotated randomly around an axis perpendicular to the deposition plan(c axis). The layered structure, with strong intra-layer covalent bonds and weak electrostatic bonds(Van der Waals bonding) between the layers, leads to a high degree of anisotropy in all properties. It is inherently brittle at room temperature.
Properties
Due to the structural differences between pyrolytic graphite and conventional commercial graphite, different properties should be expected from this material. Thermal and electrical properties:
- Linear thermal expansion
- Thermal conductivity more than four times that of copper
- Very high electrical resistance in the direction parallel to the deposition plan (perpendicular to C axis)
- Very good thermal insulation
- Very high electrical conductivity in the direction perpendicular to the deposition plan (parallel to the C axis)
From a mechanical point of view, the ultimate strength of pyrolytic graphite in stress, bending and compression increases significantly with increasing temperature. And magnetically, this material is a great diamagnetic material at room temperature(X = -4 × 10-4). Magnetic Levitation is a phenomenon that can be implemented using materials with high diamagnetic properties. This material is a suitable option for magnetic levitation due to its high diamagnetic properties.
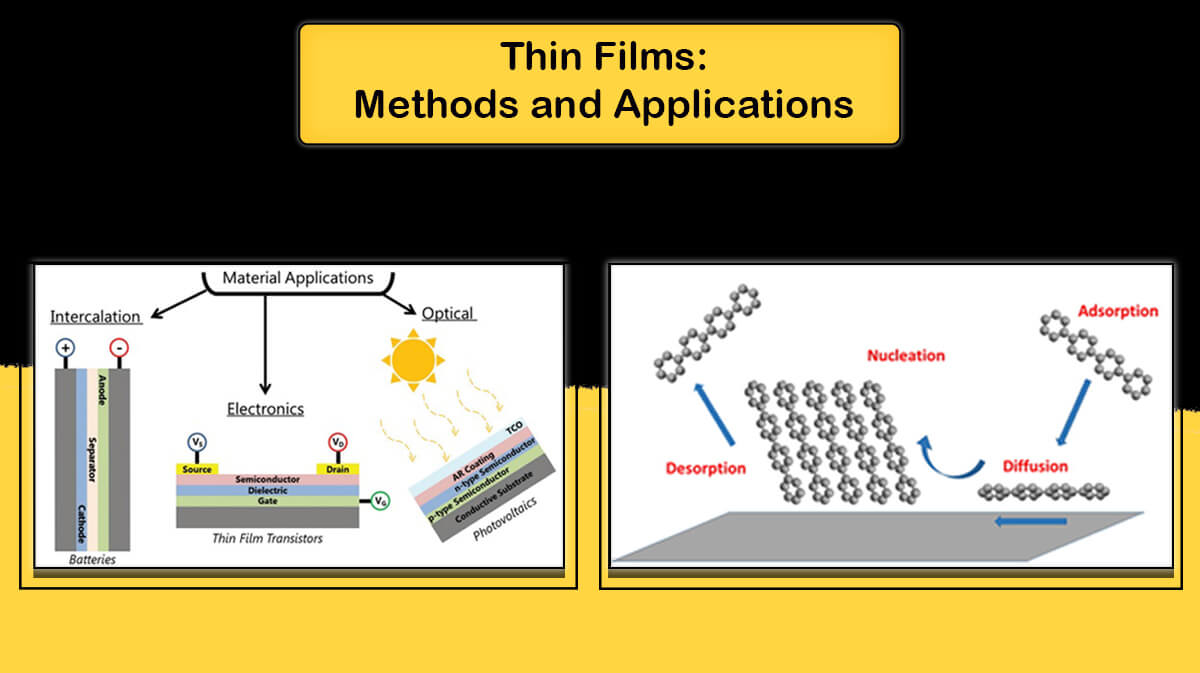
Applications
Applications of pyrolytic graphite include the following:
- Used in the rocket industry as a strong heating and cooling conductor
- Used in nuclear reactors as a neutron modulator coating
- Used in electronic devices as Heat sink
- Used in the construction of grid structures in some high power vacuum lamps
- Used in the automotive industry to create a certain amount of friction between two parts
- Used in medical engineering industries, for example in the manufacture of artificial hearts, heart valves and artificial vessels due to the lack of rapid blood clot formation
Vac Coat
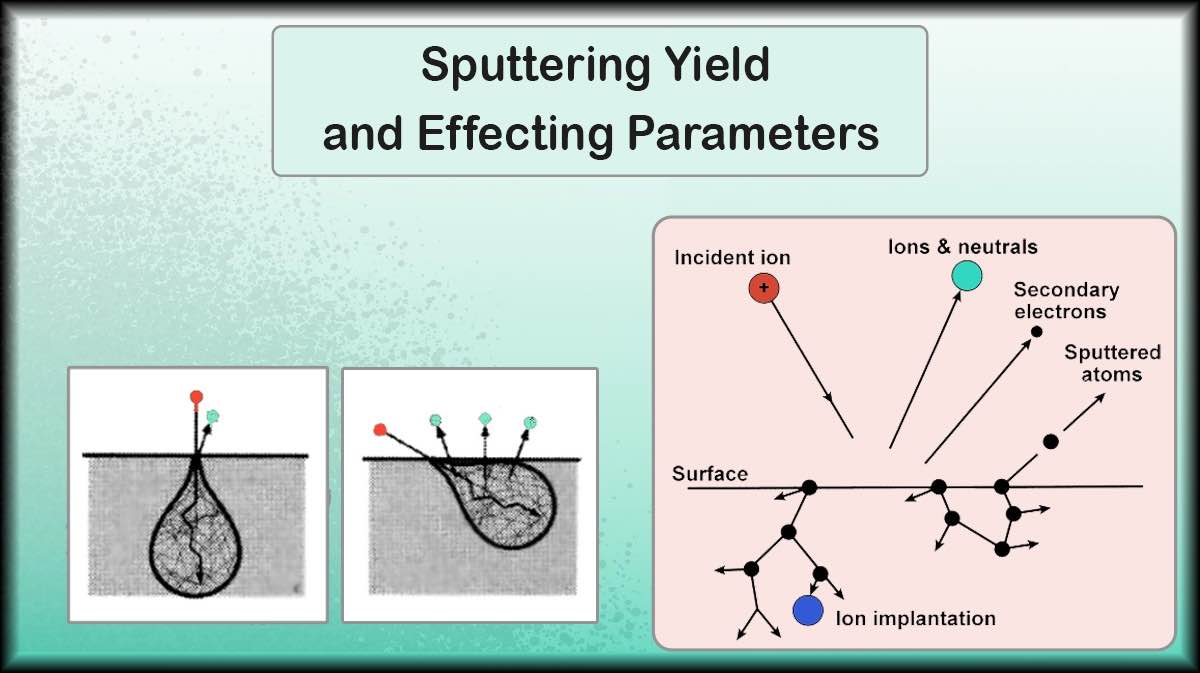
For the use of pyrolytic graphite in the thin film and semiconductor industry, this material can be deposited by sputtering method. Pyrolytic graphite is available in the market as a sputtering target. Ordinary graphite deposition using the sputtering method is difficult and time consuming due to the low sputtering yield of the graphite. Pyrolytic graphite targets are created using the CVD (Chemical Vapor Deposition) method and have a much higher density than graphite targets and less porosity. As a result, its sputtering yield is higher and Outgassing Phenomenon occurs quickly.
To create thin films of pyrolytic graphite, vacuum deposition systems made by Vac Coat Ltd. can be used in different models that are able to do deposition by sputtering method. Products of Vac Coat Ltd. that are able to perform sputtering are divided into different models according to the number of cathodes, type of power supply, chamber dimensions and ultimate vacuum. DST3, DST1-300, DSCT, DST3-T models are among the products that can deposit different materials by sputtering method. For more information about the products of this company, refer to the site.
References
- https://www.researchgate.net/publication/230346962_Properties_of_Pyrolytic_Graphite
- https://en.wikipedia.org/wiki/Pyrolytic_carbon
- https://www.lesker.com/newweb/deposition_materials/depositionmaterials_sputtertargets_1.cfm?pgid=car2#
- https://ceramics.onlinelibrary.wiley.com/doi/abs/10.1111/j.1151-2916.1961.tb11664.x
- https://arc.aiaa.org/doi/10.2514/3.2846
- https://ceramics.onlinelibrary.wiley.com/doi/abs/10.1111/j.1151-2916.1961.tb11664.x
- https://www.alliedelec.com/m/d/24f34847230e9e2134a9d1d34f665267.pdf